Polymer Synthesis 3
Mussel-Inspired Self-Polymerisation Reactions for Biomedical and Membrane Applications
Investigators:
Dr Anthony M Granville
Dr Penny Martens
Prof Martina Stenzel
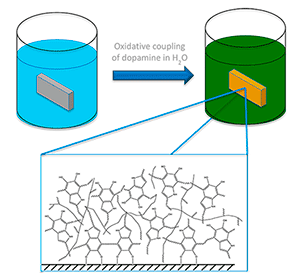
Figure 1. Schematic design for the polydopamine coating on a substrate material
Mussels and other bivalves excrete a natural polymeric adhesive shown to be water insoluble, tough, and nontoxic. This material can be synthetically manufactured using dopamine as the monomeric material in a rather simple, weakly basic, aqueous solution. The dopamine compound will cyclise to form an indole intermediate before crosslinking to form the desired polymer. When a substrate is immersed in the aqueous solutions system, the polydopamine will form a homogeneous coating on the substrate surface (Figure 1). This paves the way for simple, benign coatings of a controlled thickness to be used for drug delivery, surface modification, and metal binding for electronic applications.
Intrigued by this facile polymerisation technique, we began investigating the chemistry of this reaction to determine if other compounds similar to dopamine and its indole cyclic intermediate could be used. Recently, we’ve shown that certain indazole compounds undergo this same polymerisation and can be used in this same fashion. These compounds are of exceptional interest for their use as binding ligands for ruthenium based chemotherapy agents. When a nanospherical substrate is immersed in this dopamine/indazole aqueous system, a nanoparticle coating is generated.
Figure 2. Schematic illustration of the use of graphene oxide as surfactant in miniemulsion polymerization for synthesis of hybrid nanoparticles
Furthermore, thanks to the indazole, the coating contains attachment sites for drug loading – which is one of the key features for constructing hollow drug delivery devices (Figure 2). Since the polydopamine, and their derivative structures, contain surface hydroxyls and amines, the capsules possess surface functionality for further modifications to incorporate stealth or protein binding or target specificity. These materials represent one of the most facile and highly functional polymeric materials for the biocompatibility and drug delivery research arenas.
References
- Surface Modification of Polymeric Microspheres Using Glycopolymers for Biorecognition, A Pfaff, A. H. E. Mueller, A. M. Granville, Eur Polym J. 2011, 47, 805-815.
- Incorporation of 5-Hydroxyindazole into the Self-Polymerization of Dopamine for Novel Copolymer Synthesis, M. B. Peterson, S. P. Le-Masurier, K. Lim, J. Hook, P. Martens, A. M. Granville, Macromol. Rapid Commun. 2014, 35(3), 291 - 297.
- One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and “grafting from” RAFT polymerization, S. P. Le-Masurier, G. Gody, S. Perrier, and A. M. Granville, Polym. Chem. 2014, 5(8), 2816 - 2823.
