Polymers for Health 8
Hybrid Organic/Inorganic Nanomaterials for Imaging and Drug Delivery
Investigators:
A/Prof Cyrille Boyer
Dr May Lim (Particles and Catalysis Research Group)
Collaborator:
Prof Tom Davis (Monash)
Theranostics is the collective term used for a newly emerging field of nanotechnology where a single nanoparticle is designed to carry functionalities to instigate both diagnosis and therapy. It is now well established that nanoparticles (NP) can be designed to encapsulate/transport a wide variety of chemotherapeutic agents for delivery to cancerous (or diseased) cells. Nanoparticles can be designed to target tumors by either targeted or passive processes. Passive targeting is reliant on the size of nanoparticles being smaller than the fenestrations of endothelial cells enabling penetration of the interstitium to allow accumulation in the tumor.
The use of nanoparticles as contrast agents for biomedical in vivo imaging is also widely reported in the medical/scientific literature with many potential advantages over small molecule contrast agents. Magnetic iron oxide nanoparticles have been studied widely for applications in magnetic resonance imaging (MRI), stem cell tracking, biomolecular separation (i.e., proteins and DNA), hyperthermia and drug/gene delivery. Specifically, iron oxide nanoparticles (IONPs) have been applied clinically as MRI contrast agents for the detection of liver lesions and adenocarcinoma. In comparison to other noninvasive imaging techniques, MRI has several advantages, such as superb spatial resolution, good soft tissue contrast and zero radiation exposure.
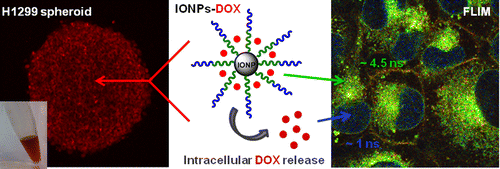
Figure 1. Iron oxide nanoparticles for the treatment of breast cancer and lung cancer.
In this project, we designed specific iron oxide nanoparticles coated with functional polymers for the specific delivery of chemotherapy drugs or gene, while the iron oxide core is employed as MRI contrast agent.
References
- Recent Developments in the Design of Nanocomposites for Photothermal and Magnetic Hyperthermia Induced Controllable Drug Delivery, Dunn, D. Dunn, M.Lim, C. Boyer, N. T. K. Thanh (2013, in press), RSC Book, NanoScience: Volume 2, 225-254, DOI: 10.1039/9781849737623-00225.
- Magnetic Nanoparticles with Diblock Glycopolymer Shells give Lectin Concentration-Dependent MRI Signals and Selective Cell Uptake, Johan S. Basuki, Lars Esser, Hien T. T. Duong, Qiang Zhang, Paul Wilson, Michael R. Whittaker, David M. Haddleton, Cyrille Boyer, Thomas P. Davis, Chemical Science, Accepted on 8th November 2013.
- Using Fluorescence Lifetime Imaging Microscopy (FLIM) to Monitor Theranostic Nanoparticle Uptake and Intracellular Doxorubicin Release J. Basuki, H. T.T. Duong, A. Macmillan, R.Erlich, L. Esser, M. Akerfeldt, R. Whan, M. Kavallaris, C. Boyer, T.P. Davis (in press) ACS Nano, accepted on 14/10/2013.
- Functional Iron Oxide Magnetic Nanoparticles with Hyperthermia Drug Release Ability using a Combination of Orthogonal Click Chemistries T. T. T. N’Guyen, H. T. T. Duong, J. Basuki, V. Montembault, S. Pascual, C. Guibert, J. Fresnais, C. Boyer, M. R. Whittaker, T. P. Davis, and L. Fontaine (in press) Angewandte Chemie – International Edition, accepted on 12/10/2013.
- Grafting of P(OEGA) Onto Magnetic Nanoparticles Using Cu(0) Mediated Polymerization: Comparing Grafting “from” and “to” Approaches in the Search for the Optimal Material Design of Nanoparticle MRI Contrast Agents, J.S. Basuki, L. Esser, P.B. Zetterlund, M.R. Whittaker, C. Boyer, T.P. Davis (2013) Macromolecules, 46 (15), pp 6038–6047.
- Polymer-Grafted, Nonfouling, Magnetic Nanoparticles Designed to Selectively Store and Release Molecules via Ionic Interactions, J. S. Basuki, H. T. T. Duong, A. Macmillan, R. Whan, C. Boyer, T. P. Davis (2013) Macromolecules, in press, DOI: 10.1021/ma401171d
- The Design And Utility Of Polymer Stabilized Iron Oxide Nanoparticles For Nanomedicine Applications, C.Boyer, M.R.Whittaker, V.Bulmus, J.Liu, T.P.Davis (2010) NPG Asia Materials (Nature Publisher Group) 2, 23-30.
- (2010) Anti-Fouling, Magnetic Nanoparticles For siRNA Delivery. C.Boyer, P.Priyanto, T.P.Davis, V.Bulmus, D.Pissuwan, V.Bulmus, M.Kavallaris, W.Y.Teoh, R.Amal, M.Carroll, R.Woodward, T. St Pierre Journal of Materials Chemistry 20, 255-265.
